How to Calculate the Amount of P-nitrophenol Produced
In my test tube I have 19mL of glycine buffer and 01mL of p-nitrophenol and then I am suppose to calculate how much p-nitrophenol in nmol is present. Working stock solution of 1mM pNP is.
P Nitrophenol Standard Curve 2 9 Characterization Of Alkaline Download Scientific Diagram
Determination of p-nitrophenol produced using a high and a low substrate concentration based on p-nitrophenol standards.

. You start with 01 ml of 025mM 250 uM or 250000 nM p-NP final volume is 2 ml so you diluted it 20-fold. Mix the contents of each tube well. In this case the amount of p-Nitrophenol in a sample with an absorbance value of 073 is approximately 44 nmol.
Calculate the nmol of p-nitrophenol produced in your reactions using Beers law A εcl and mathematical manipulation in the following way - divide the value obtained for absorbance by the extinction co-efficient of p-nitrophenol at 405 nm 102 mM-1 cm-1 and the path length of the cuvette 1 cm c Ael- you obtain the value for c in mM mmol l-1-then you multiply this. When at high enzyme concentration almost all the enzyme was produced by the first two minutes and staying steady through 8. Starting with the theoretical approach the key equation is A e c l where e is the molar extinction coefficient c is the concentration M and l is the path length measured in cm.
Add 0lM NaOH to these cuvettes as indicated in Table A. Can someone please help. USCG 1999 CAMEO Chemicals.
1 nmol 1 nanomole 1 x 10 9 mol 0000000001 mol Table 15. But at Low concertation the production of p-nitrophenol continued to occur through 8 minutes. P-nitrophenol MW 13911 10mM pNP stock solution is prepared by dissolving 00139 g of pNP in 10 ml of desired buffer.
U can calculate by two ways first by making standard graph of p-nitrophenol and by measuring abortion at 405 or 410 by using extension coefficient. It is a conjugate acid of a 2-nitrophenolate. 005m glycine buffer pH 95 025mM p-nitrophenol in 005M glycine buffer pH 95 02M NaOH.
Level of Experiment This practical is aimed at second year first semester introductory biochemistry students. These labels indicate the concentration of p-nitrophenol in units of nmolesml. Multiply l by c and then divide A by the product to solve for molar absorptivity.
2-nitrophenol is a yellow solid. To determine the amount of product corresponding to this value locate the absorbance value of 073 approximately on the y-axis and then follow the value horizontally until it intersects with the standard curve. Biological knowledge is growing explosively and recent advances have fundamentally changed our understanding of life processes.
If you do not have a spectrophotometer skip to Analysis of Results. Then according to the enzyme unit definition you can calculate. Follow this process to create a standard curve with your standards and then use that standard curve to analyze your samples.
It has a role as a human xenobiotic metabolite and a mouse metabolite. Then calculate the delta Atime unit from the linear part of the graph. The amount of product p-nitrophenol is determined by reading the absorbance at 405 nm and using a molar extinction coefficient of 18000 M-1 cm-1 16000 M-1cm-1 for 05 M EDTA 1 3.
It may include a variety of diverse activities such as the sequencing of DNA determining the rate of neuron activity or measuring plant diversity in a prairie. This is the first of 2 experiments on enzyme. You need to draw the absorbance changes against the time.
Biology is the science of life and life processes. One unit of the protein phosphatase activity is defined as the amount of enzyme that hydrolyzes 1 nanomole of PNPP in one minute at 30C in a total reaction. Then you make the difference 0244-0157 0087 and you have to convert this into phosphate produced applying the Beer Lamber equation 0087 c.
Amount of p-Nitrophenol nmol Time min Cuvette Absorbance. Sinks in and mixes slowly with water. From this point draw a line down to the x-axis amount of p-nitrophenol and read.
Then a line was drawn down to the x-axis to find the amount of p-Nitrophenol. In activity four the testing on how enzyme concentration affected the overall amount of p-nitrophenol produces at each tested time. It is a conjugate acid of a 4-nitrophenolate.
Using the values you obtained for A c and l plug them into the equation ɛ Alc. Plug in the values for the variables and solve the equation for molar absorptivity. The final concentration will be 250000 nm2012500 nM.
4-Nitrophenol is used to manufacture drugs fungicides. 2-nitrophenol is a member of the class of 2-nitrophenols that is phenol in which one of the hydrogens that is ortho to the hydroxy group has been replaced by a nitro group. To plot the amount of p-nitrophenol produced versus time and to use this plot in calculating the specific activity of acid phosphatase under these conditions.
0 5 l0 20 30 40 50 60. Table A Tube Volume 60Volume 01M NaOH M -NP. 4-nitrophenol is a member of the class of 4-nitrophenols that is phenol in which the hydrogen that is para to the hydroxy group has been replaced by a nitro group.
Add 60 M -nitrophenol to the cuvettes as indicated in Table A.

Shows The Standard Graph Of P Nitrophenol For Determining The Alkaline Download Scientific Diagram
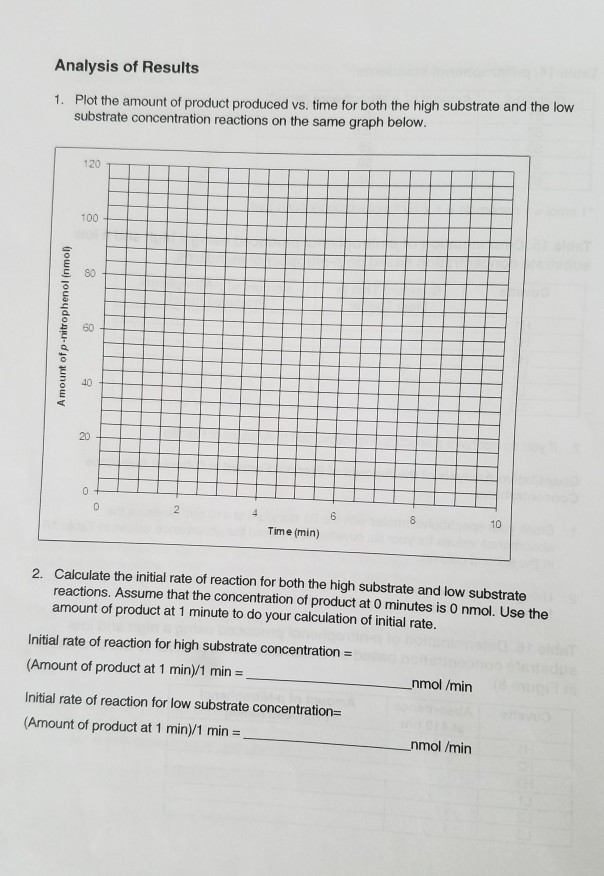
Table 14 Pnitrophenol Standards Standardーーamount Of Chegg Com
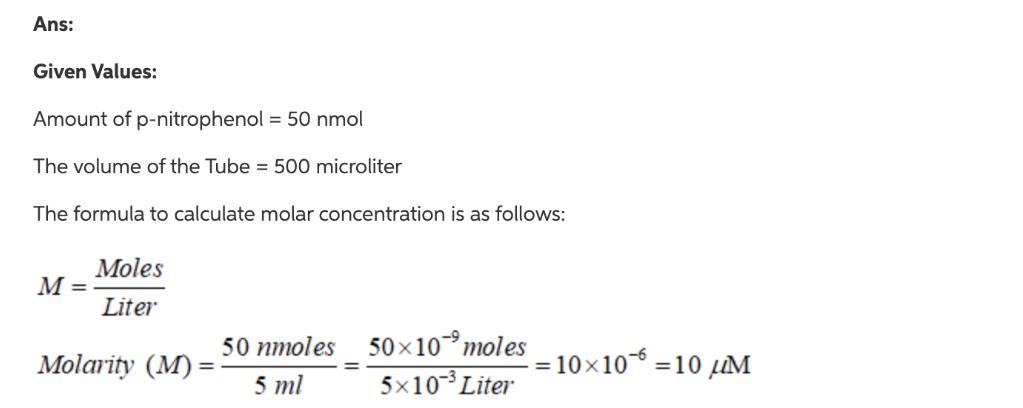
Solved Calculate The Concentration Of P Nitrophenol Um And Chegg Com

A Standard Linear Curve Of P Nitrophenol And Phthalic Acid B Esterase Download Scientific Diagram

Standard Curve For P Nitro Phenol Download Scientific Diagram
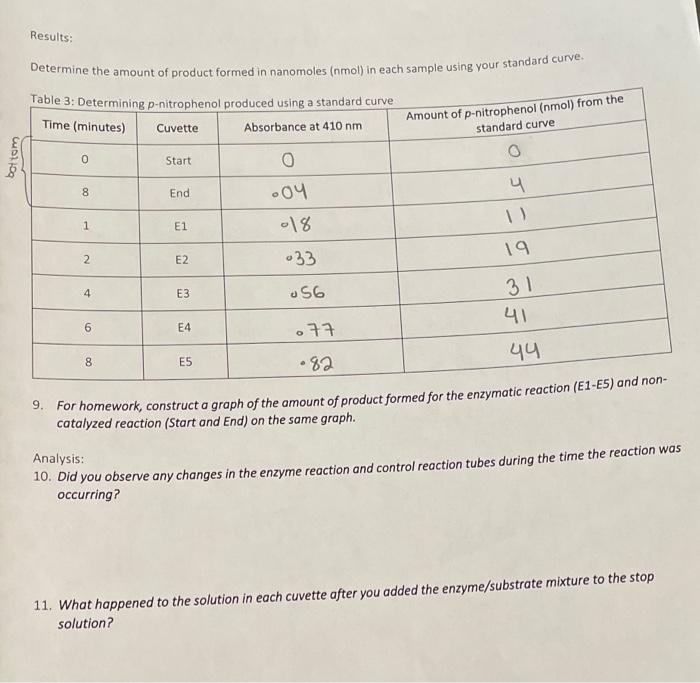
Solved Results Determine The Amount Of Product Formed In Chegg Com

Solved Table 14 P Nitrophenol Standards Standard Amount Ol P Nltrophenol Nmolt Nmol Nanomole X10 9 Mol 0 Oooooo001 Mol Table 15 Determination Of Substrate P Nitrophenol Produced Using A Concentration High And Low Based On P Nitrophenol Standards
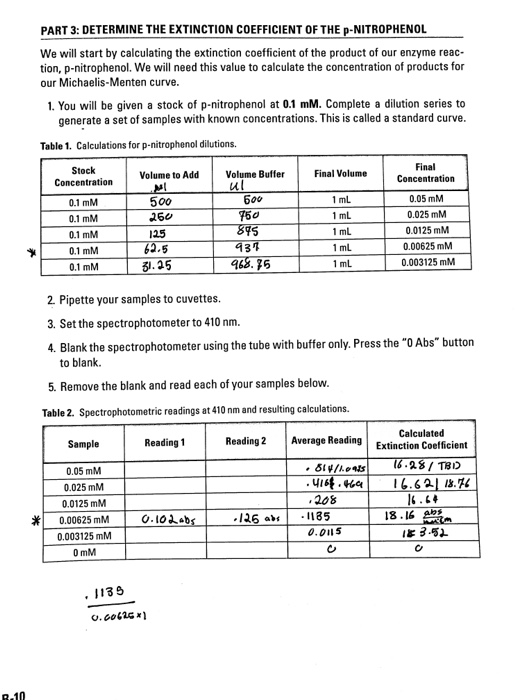
Solved Part 3 Determine The Extinction Coefficient Of The Chegg Com
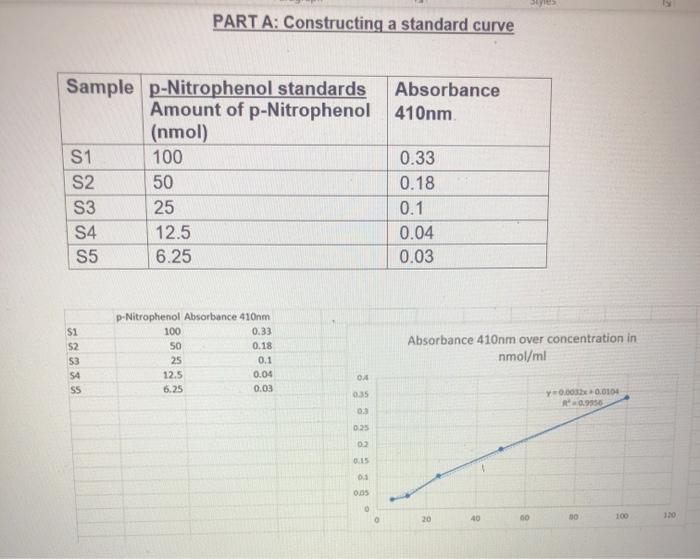
Part A Constructing A Standard Curve Absorbance Chegg Com
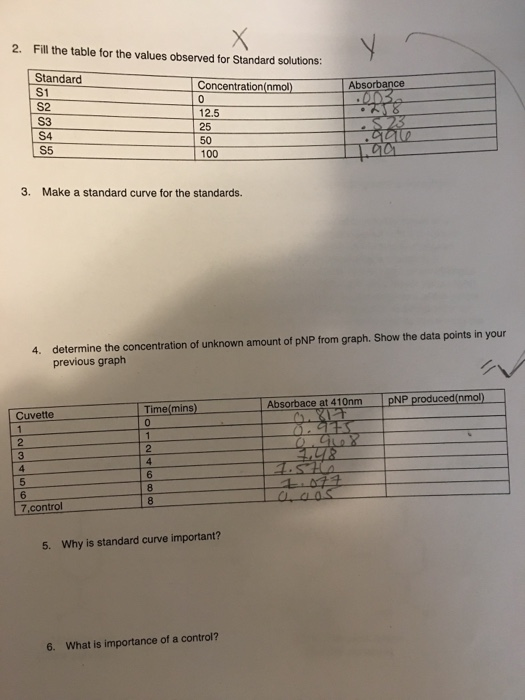
Solved Making Of P Nitrophenol Standards 1 2 Solution S5 Chegg Com
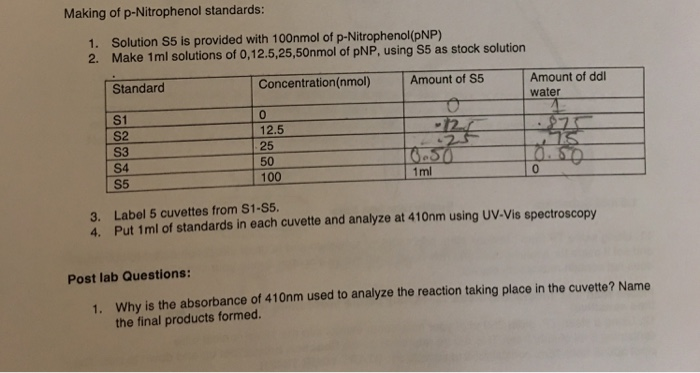
Solved Making Of P Nitrophenol Standards 1 2 Solution S5 Chegg Com
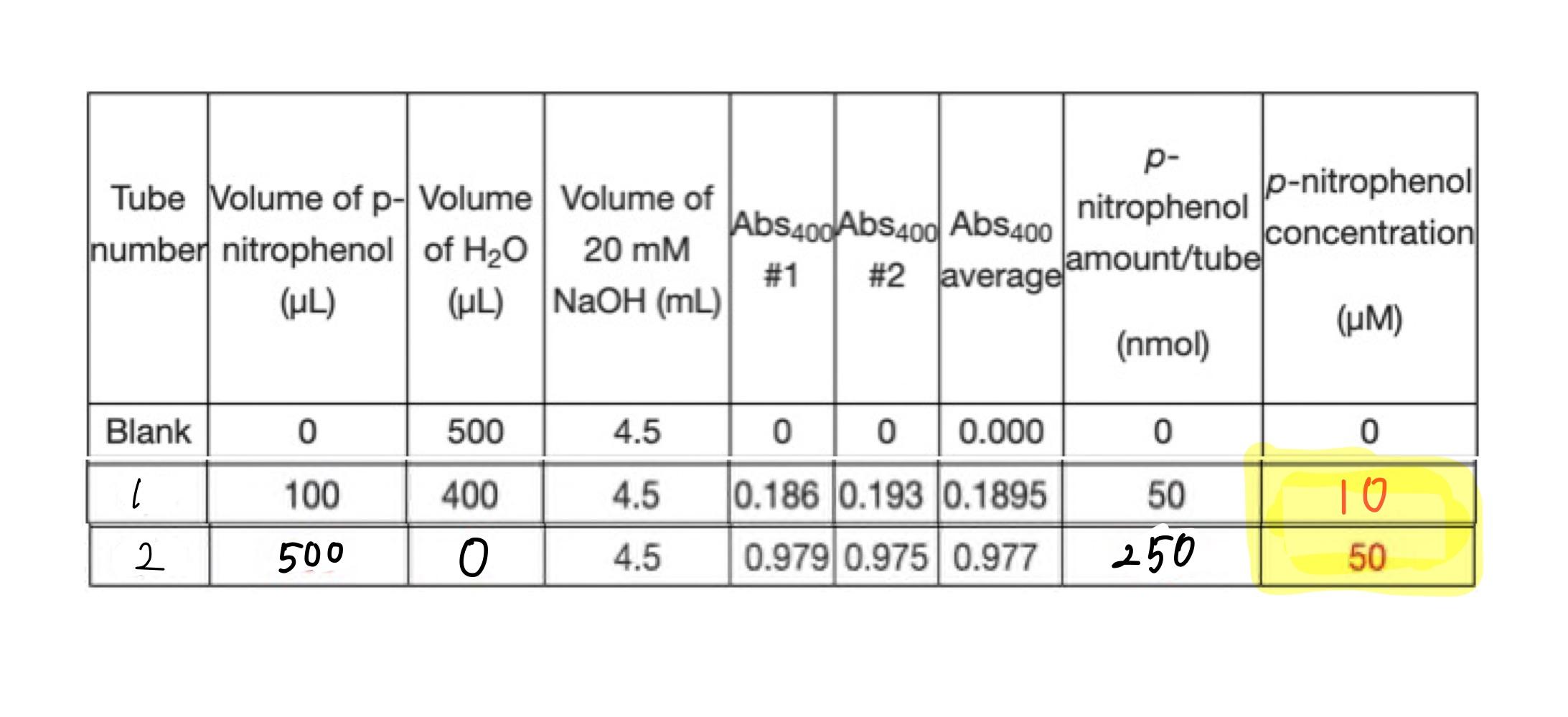
Solved Calculate The Concentration Of P Nitrophenol Um And Chegg Com
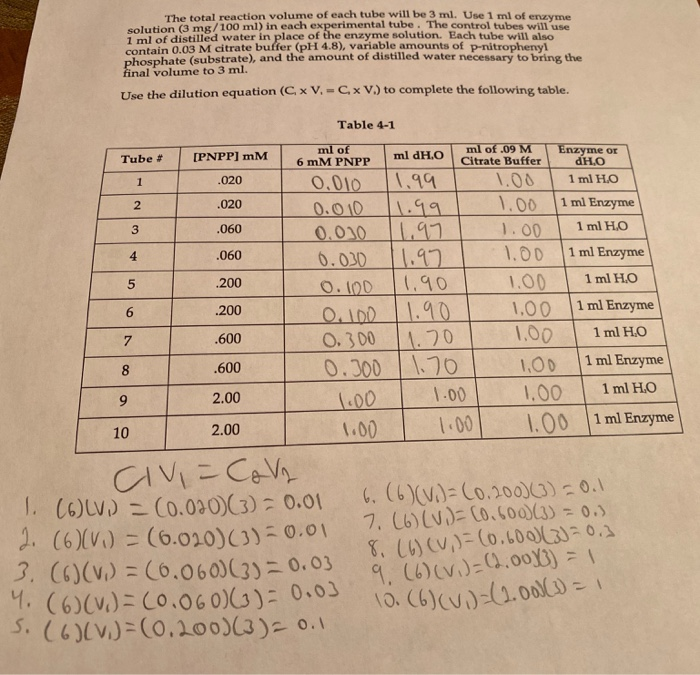
Solved Making Of P Nitrophenol Standards 1 2 Solution S5 Chegg Com

Calibration Curve Of P Nitrophenol Download Scientific Diagram

Standard Graph Of Pnp Curve Of Opp Enzyme Liberating 1 M P Nitrophenol Download Scientific Diagram
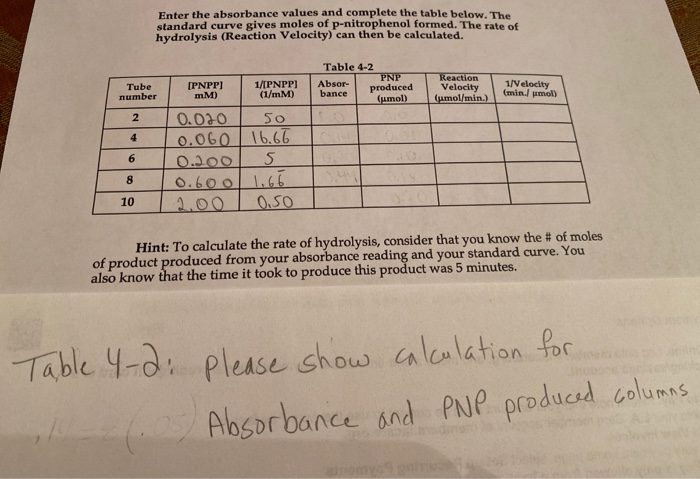
Standard Curve Data Umol P Nitrophenol Absorbance 0 0 Chegg Com

Standard Curve For P Nitrophenol Download Scientific Diagram
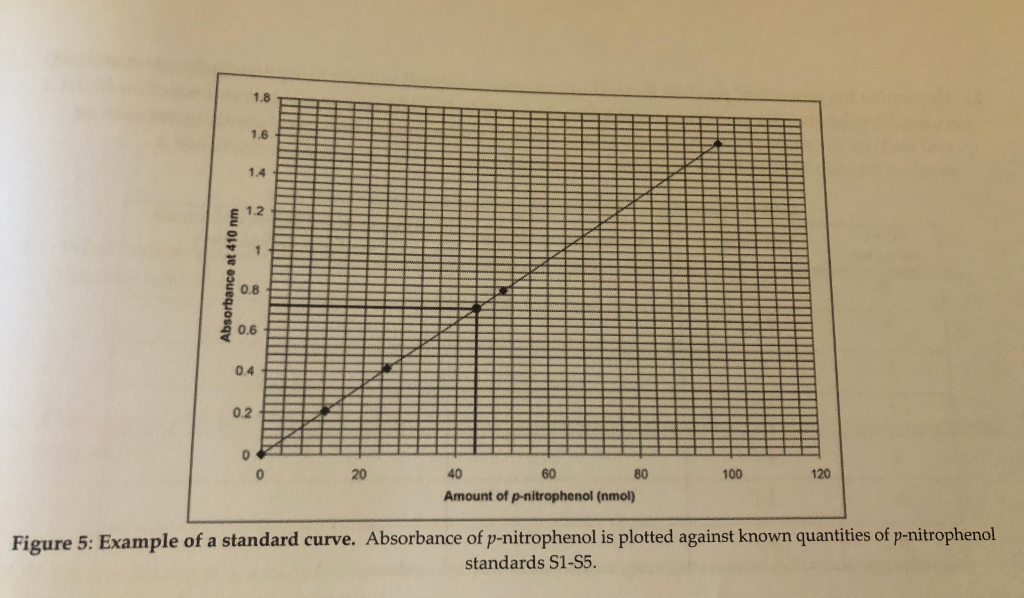
Solved 1 8 1 6 1 4 1 2 0 8 0 6 0 4 0 2 20 40 60 80 100 120 Chegg Com
Standard Curve Data Umol P Nitrophenol Absorbance 0 0 Chegg Com
Comments
Post a Comment